Aptima® Multitest swab
Women’s health testing made simple with the Aptima® Multitest Swab.
Flexibility
Can be used to collect samples from a variety of specimen collection sites.1
- Vaginal swab
- Throat swab
- Rectal swab
- Penile meatal swab
- Genital lesion swab for HSV
Versatility
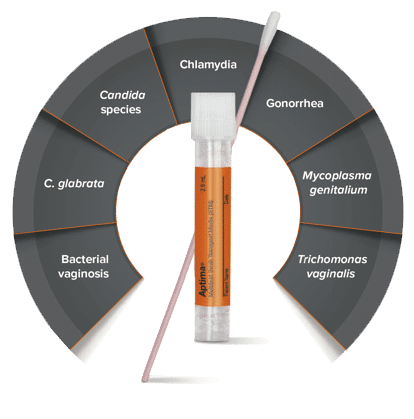
Detect up to SEVEN infections and disease states with just one vaginal swab sample.2,3,4,5
- Chlamydia
- Gonorrhea
- Mycoplasma genitalium
- Trichomonas vaginalis
- Bacterial vaginosis
- Candida species
- C. glabrata
Convenience
- Reduce patient callbacks for re-collection when reflex or add-on testing is ordered.
- A single swab simplifies sample collection and streamlines office inventory.
Test your knowledge
A vaginal sample provides more accuracy than a urine sample.
Women prefer urine collection over a vaginal swab.
Vaginal swab is the CDC’s preferred collection method.
The Aptima® Multitest Swab can be used to collect samples from a variety of specimen collection sites.
The Aptima® Multitest Swab allows for retesting the sample for other infections and disease states.
The Aptima® Multitest Swab detects up to 7 infections or disease states at a time.7-10
A vaginal sample provides more accuracy than a urine sample.
Urine samples can miss up to 10% of underlying infections where vaginal samples miss fewer than 1% of infections.*1-2,3
*Refer to individual assay package inserts for cleared specimen types and performance claims. References: 1. Chernesky M, et al. Head-to-head comparison of second-generation nucleic acid amplification tests for detection of Chlamydia trachomatis and Neisseria gonorrhoeae on urine samples from female subjects and self-collected vaginal swabs. J Clin Microbiol. 2014 Jul;52(7):2305-10. doi: 10.1128/JCM.03552-13. Epub 2014 Apr 2. PMID: 24696024; PMCID: PMC4097753. 2. Van Der Pol B, et al. Combined Testing for Chlamydia, Gonorrhea, and Trichomonas by Use of the BD Max CT/GC/TV Assay with Genitourinary Specimen Types. J Clin Microbiol. 2016 Dec 28;55(1):155-164. doi: 10.1128/JCM.01766-16. PMID: 27795343; PMCID: PMC5228226. 3. Aptima Trichomonas vaginalis Assay [package insert]. 503684-IFU-PI, San Diego, CA; Hologic, Inc., 2021.
A vaginal sample provides more accuracy than a urine sample.
Urine samples can miss up to 10% of underlying infections where vaginal samples miss fewer than 1% of infections.*1-2,3
*Refer to individual assay package inserts for cleared specimen types and performance claims. References: 1. Chernesky M, et al. Head-to-head comparison of second-generation nucleic acid amplification tests for detection of Chlamydia trachomatis and Neisseria gonorrhoeae on urine samples from female subjects and self-collected vaginal swabs. J Clin Microbiol. 2014 Jul;52(7):2305-10. doi: 10.1128/JCM.03552-13. Epub 2014 Apr 2. PMID: 24696024; PMCID: PMC4097753. 2. Van Der Pol B, et al. Combined Testing for Chlamydia, Gonorrhea, and Trichomonas by Use of the BD Max CT/GC/TV Assay with Genitourinary Specimen Types. J Clin Microbiol. 2016 Dec 28;55(1):155-164. doi: 10.1128/JCM.01766-16. PMID: 27795343; PMCID: PMC5228226. 3. Aptima Trichomonas vaginalis Assay [package insert]. 503684-IFU-PI, San Diego, CA; Hologic, Inc., 2021.
Women prefer vaginal swab over urine collection.
60% of women prefer vaginal swab over urine collection.4
4. Chernesky MA, Hook EW, Martin DH, et al. Women find it easy and prefer to collect their own vaginal swabs which are effective for the diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae. Sex Transm Dis.2005;32(12):729-33.
Women prefer vaginal swab over urine collection.
60% of women prefer vaginal swab over urine collection.4
4. Chernesky MA, Hook EW, Martin DH, et al. Women find it easy and prefer to collect their own vaginal swabs which are effective for the diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae. Sex Transm Dis.2005;32(12):729-33.
Vaginal swab is the CDC’s preferred collection method.
For female screening, specimens obtained with a vaginal swab are the preferred specimen type. Vaginal swab specimens are as sensitive as cervical swab specimens and there is no difference in specificity.5
5. CDC. Recommendations for the Laboratory-Based Detection of Chlamydia trachomatis and Neisseria gonorrhoeae. MMWR Recomm Rep.2014;632. https://www.cdc.gov/std/laboratory/2014labrec/2014-lab-rec.pdf
Vaginal swab is the CDC’s preferred collection method.
For female screening, specimens obtained with a vaginal swab are the preferred specimen type. Vaginal swab specimens are as sensitive as cervical swab specimens and there is no difference in specificity.5
5. CDC. Recommendations for the Laboratory-Based Detection of Chlamydia trachomatis and Neisseria gonorrhoeae. MMWR Recomm Rep.2014;632. https://www.cdc.gov/std/laboratory/2014labrec/2014-lab-rec.pdf
The Aptima® Multitest Swab can be used to collect samples from a variety of specimen collection sites.
Vaginal, throat, rectal, penile meatal and genital lesion for HSV.†6
†The label includes a line for specimen source to identify the site of the patient sample. 6. Aptima Multitest Swab [package insert], AW-14413. San Diego, CA, Hologic, Inc.; 2019.
The Aptima® Multitest Swab can be used to collect samples from a variety of specimen collection sites.
Vaginal, throat, rectal, penile meatal and genital lesion for HSV.†6
†The label includes a line for specimen source to identify the site of the patient sample. 6. Aptima Multitest Swab [package insert], AW-14413. San Diego, CA, Hologic, Inc.; 2019.
The Aptima® Multitest Swab allows for retesting the sample for other infections and disease states.
The Aptima Multitest Swab allows for re-collection when reflex or add-on testing is ordered, which simplifies sample collection and decreases patient callbacks.
7. CDC. Recommendations for the Laboratory-Based Detection of Chlamydia trachomatis and Neisseria gonorrhoeae. MMWR Recomm Rep.2014;632. https://www.cdc.gov/std/laboratory/2014labrec/2014-lab-rec.pdf 8. Aptima Multitest Swab [package insert], AW-14413. San Diego, CA, Hologic, Inc.; 2019
The Aptima® Multitest Swab allows for retesting the sample for other infections and disease states.
The Aptima Multitest Swab allows for re-collection when reflex or add-on testing is ordered, which simplifies sample collection and decreases patient callbacks.
7. CDC. Recommendations for the Laboratory-Based Detection of Chlamydia trachomatis and Neisseria gonorrhoeae. MMWR Recomm Rep.2014;632. https://www.cdc.gov/std/laboratory/2014labrec/2014-lab-rec.pdf 8. Aptima Multitest Swab [package insert], AW-14413. San Diego, CA, Hologic, Inc.; 2019
The Aptima® Multitest Swab detects up to 7 infections or disease states at a time.7-10
Chlamydia, Gonorrhea, Mycoplasma genitalium, Trichomonas vaginalis, Candida species, C. glabrata, Bacterial vaginosis.
7. Aptima Combo 2 Assay [package insert]. 502446-IFU-PI, San Diego, CA; Hologic, Inc., 2021. 8. Aptima Mycoplasma genitalium assay [package insert]. San Diego, CA; Hologic, Inc., 2021. 9. Aptima CV/TV assay [package insert]. AW-18812, San Diego, CA; Hologic, Inc., 2021. 10. Aptima BV assay [package insert]. AW-18811, San Diego, CA; Hologic, Inc., 2021.
The Aptima® Multitest Swab detects up to 7 infections or disease states at a time.7-10
Chlamydia, Gonorrhea, Mycoplasma genitalium, Trichomonas vaginalis, Candida species, C. glabrata, Bacterial vaginosis.
7. Aptima Combo 2 Assay [package insert]. 502446-IFU-PI, San Diego, CA; Hologic, Inc., 2021. 8. Aptima Mycoplasma genitalium assay [package insert]. San Diego, CA; Hologic, Inc., 2021. 9. Aptima CV/TV assay [package insert]. AW-18812, San Diego, CA; Hologic, Inc., 2021. 10. Aptima BV assay [package insert]. AW-18811, San Diego, CA; Hologic, Inc., 2021.
Congratulations, you got 100%!
Very impressive. We can tell youthis is a rare accomplishment, and not something we see very often.
Share with a colleague to see if they know as much as you.
XX%! Not a perfect score,
but you learned something new!
You are in good company; we rarely see anyone get 100%.Share with a colleague to see if they know as much as you.
Take this quick 6 questions true/false quiz and find out.
Vaginal swab is the CDC’s preferred collection method for chlamydia and gonorrhea.6
The Aptima® Multitest Swab Specimen Collection Kit is indicated for use with the following assays:
FDA-Cleared Assays
- Aptima Combo® 2 Assay (CT/NG)
- Aptima® Mycoplasma genitalium Assay
- Aptima® Trichomonas vaginalis Assay
- Aptima® HSV 1 & 2 Assay
- Aptima® BV Assay
- Aptima® CV/TV Assay
“Self-collected vaginal swab specimens are equivalent in sensitivity and specificity to those collected by a clinician using NAATs, and women find this screening strategy highly acceptable.”7