Aptima® Mycoplasma genitalium Test
Mycoplasma genitalium (M. gen) is a highly prevalent STI
Testing for M. gen is recommended by the Centers for Disease Control and Prevention (CDC) for patients with recurrent nongonoccal urethritis (NGU), recurrent cervicitis, and Pelvic Inflammatory Disease (PID).1 Both women and men with M. gen infections can be asymptomatic and when left untreated, this infection can result in serious health consequences.1
Women
- Frequently asymptomatic1
- Detected in 10%-30% of women with clinical cervicitis1
- Prevalence of M. genitalium is identified in up to 22% of pelvic inflammatory disease (PID) cases1
- Untreated PID can lead to adverse pregnancy outcomes1
Men
- Responsible for 40% of persistent or recurrent urethritis in men1
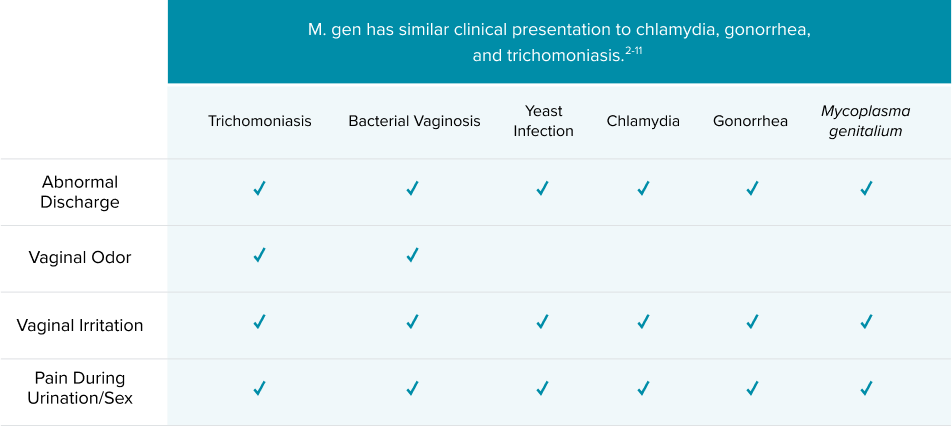
Proper treatment depends on proper diagnosis
The treatment for each infection is organism-specific.1 An accurate diagnosis is critical to ensuring infections are treated successfully.
Recommended treatments from CDC STI Treatment Guidelines 2021 are organism-specific
Chlamydia1
| Gonorrhea1
* For persons weighing ≥150 kg, 1 g Ceftriaxone should be administered. | Trichomoniasis1
|
Treatment considerations
M. GEN. 1A two-stage therapy approach accompanied with resistance testing, if available, is recommended for treating M. gen. 1
|
The content in this piece is for information purposes only and is not intended to be medical advice.
The Aptima® Mycoplasma genitalium assay, a Nucleic Acid Amplification Test (NAAT), has up to 100% sensitivity.12‡ The Aptima® assay targets rRNA and out-performed DNA tests.13-14*†
M. gen infection contains a very low organism load compared to other infections.15 Peer-reviewed article reported that the rRNA-based Aptima M. gen assay had higher clinical sensitivity compared to that of DNA-based assay.13-14 The CDC recommends NAATs for detection of M. gen.1
Sensitivity of Detection in Patients with M. gen Infections:12-14
%
Aptima® M. genitalium assay RNA-based test
%
Aptima® M. genitalium assay RNA-based test
%
DNA-based LDT test
%
DNA-based test
*Sensitivity from peer-reviewed article Le Roy et al. study
†Sensitivity from peer-reviewed Unemo et al. study
‡Sensitivity information provided above is specific to patient-collected
vaginal swab (PVS) for symptomatic patients. For complete performance
characteristics for the Aptima Mycoplasma genitalium assay, please refer to
the package insert.
†Sensitivity from peer-reviewed Unemo et al. study
‡Sensitivity information provided above is specific to patient-collected
vaginal swab (PVS) for symptomatic patients. For complete performance
characteristics for the Aptima Mycoplasma genitalium assay, please refer to
the package insert.
Contact Us
We are here to support you. Have a question or need to talk to a Hologic team member?