Aptima® HPV 16 18/45
Genotype Assay
A Targeted Approach

“We confirmed the utility of 16/18 genotyping in cervical cancer screening strategies, while pooled detection of non-16/18 genotypes is sufficient”
– Monsonego, et al., ATHENA trial2
ASCCP Management Genotyping Guidelines3*
“Ultimately, the placement of genotypes into risk strata will require consensus from multiple prospective and observational studies and modeling studies that provide risk estimates and corresponding number of colposcopy referrals in order to provide an accurate risk-benefit analyses.”
– Stoler, et al.4
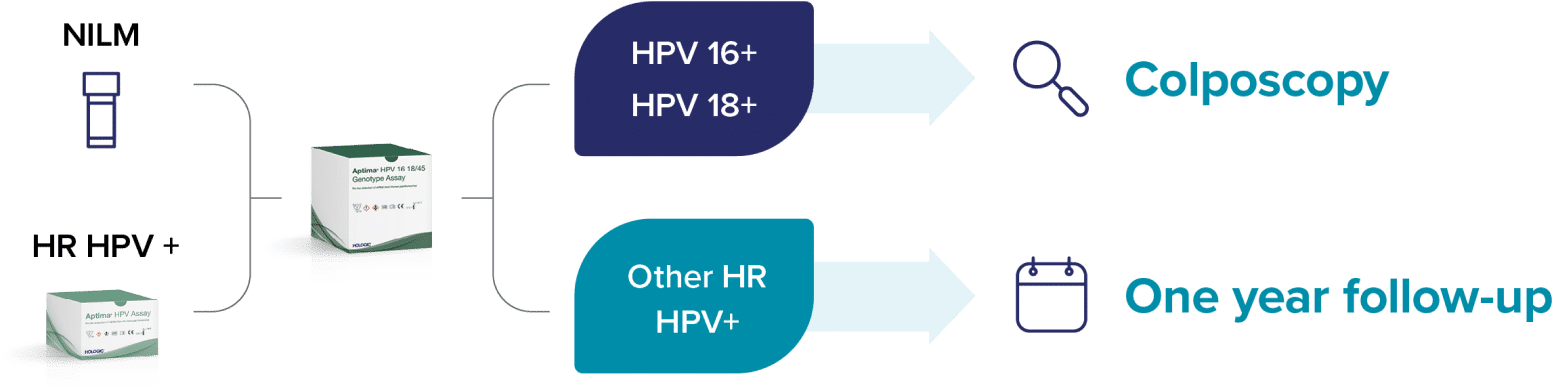
“Including additional genotypes to HPV 16/18 for immediate colposcopy in HPV primary screening algorithms is ultimately a question of balance: do additional colposcopies represent a significant benefit to patients who would otherwise return in 1 year for a follow-up versus the risk of loss to follow-up and lack of treatment of prevalent CIN2/3.”
– Stoler, et al.4
*Full genotyping recommendations can be found in the ASCCP app.
HPV Clearance and Progression
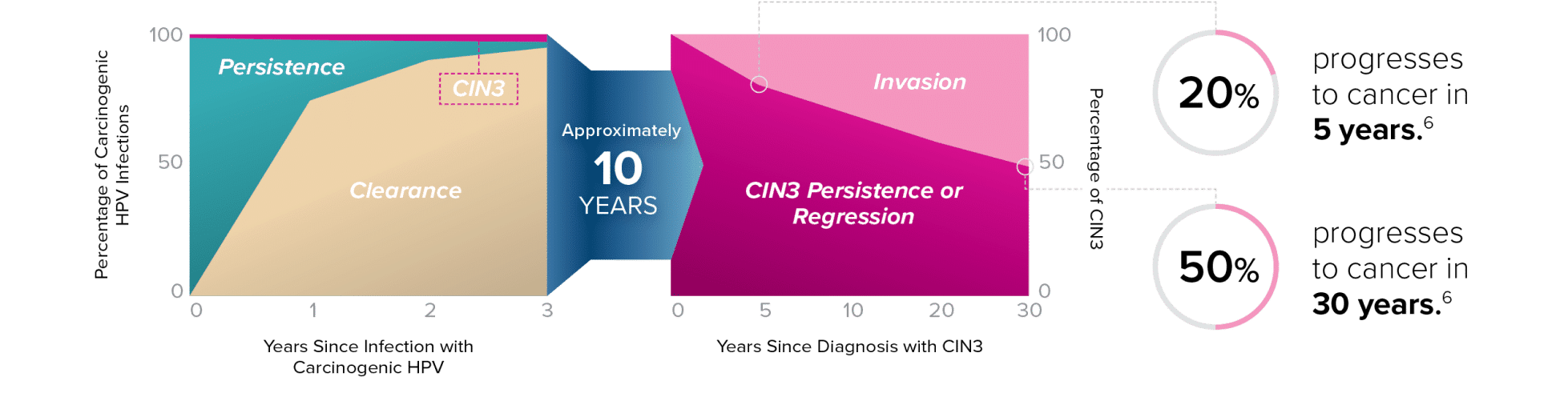
“. . . . many cases of CIN3/AIS would not progress to cancer if left untreated . . . . Therefore, true cancer risk posed by various HPV types can be misspecified even when estimated by the prospective risk of CIN3/AIS . . . .”
– Demarco, et al.7
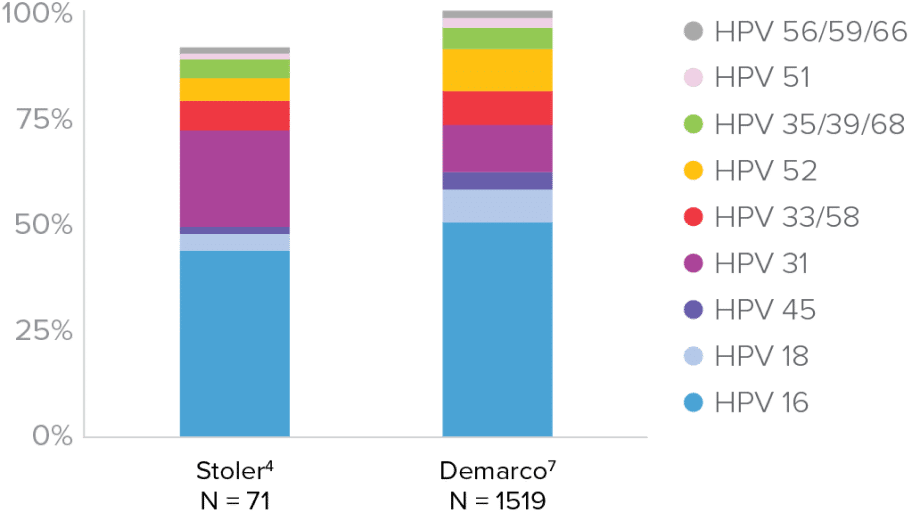
HPV Genotypes in Cases of CIN3+ and Cervical Cancer
CIN3+ Cases by Genotype
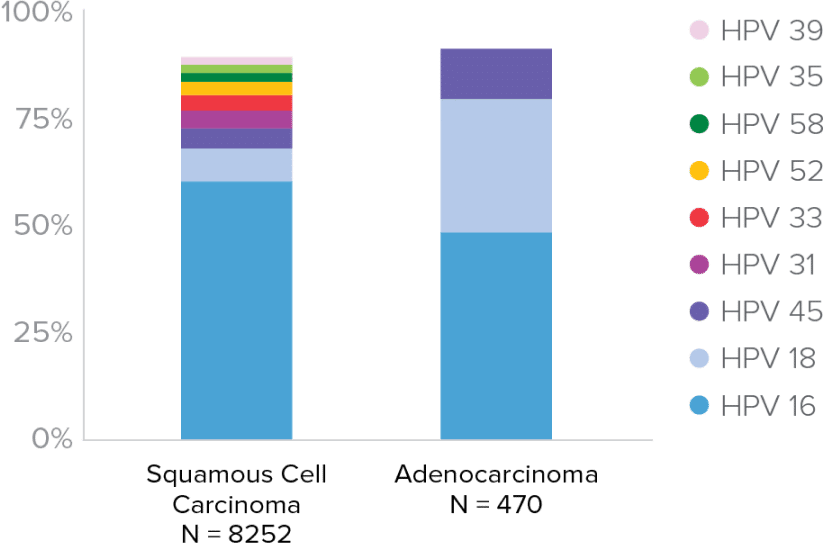
HPV type 16 associated with8:
- 62% of Squamous Cell Carcinomas
- 50% of Adenocarcinomas
HPV types 16, 18, 45 associated with8:
- Up to 75% of Squamous Cell Carcinomas
- 94% of HPV-related cervical adenocarcinomas
- Third most common HPV type in invasive cervical cancer
- Identifies more women at risk for adenocarcinoma, with minimal impact to colposcopy