ThinPrep® Pap Test
A wealth of knowledge in a single vial1,2
The first liquid-based Pap test with FDA approval/clearance for sample collection of Pap, HPV, chlamydia/gonorrhea, and trichomoniasis testing from the same vial.
FDA Approved
- ThinPrep® Pap test
- Aptima® HPV assay
- Aptima® HPV 16 18/45
genotype assay - cobas® HPV test
- cobas® AMPLICOR CT/NG test
- Hybrid Capture® 2 HPV test
FDA Cleared
- Aptima Combo 2® assay
- Aptima® Trichomonas
vaginalis assay - ProbeTec® Chlamydia
trachomatis (CT) test - ProbeTec® Neisseria
gonorrhoeae (GC) test
The ThinPrep® Pap Test collection process provides:
Patient Comfort
Out-of-the-vial testing minimizes
the number of samples required
for multiple test results.
Clinician Versatility
Multiple FDA-approved ancillary
testing options available from a single vial.
Chain-of-Custody Verification
Sample integrity and preservation

Collection device rinsed
in ThinPrep vial.
Full contents of vial preserved
for use in slide presentation.
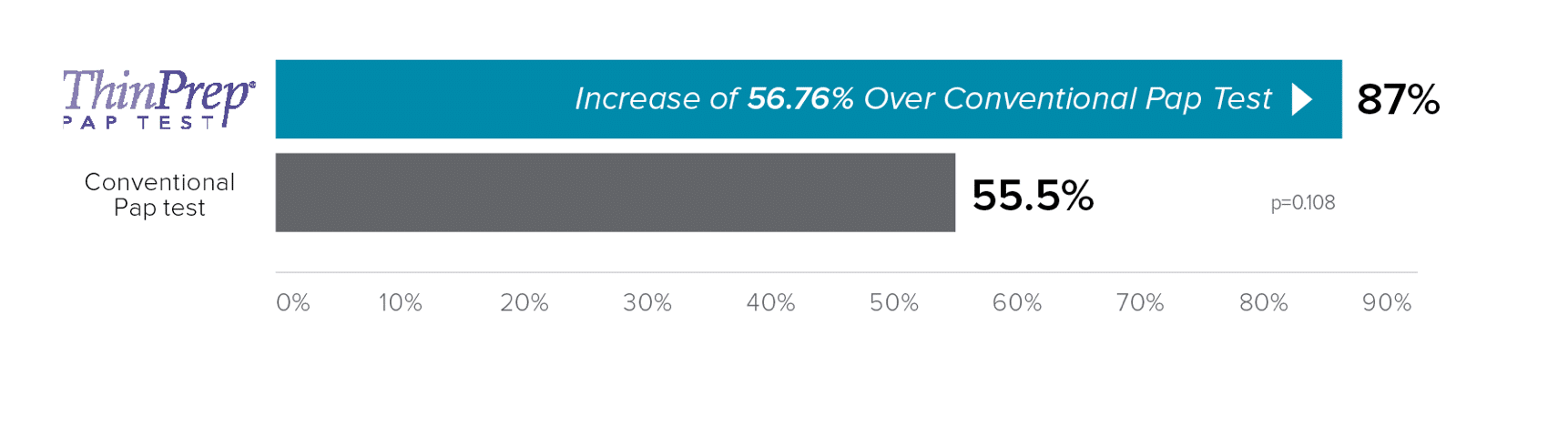
Raising the bar in Pap testing results
Studies have reported reductions in unsatisfactory results when the ThinPrep® imaging system was implemented.3,4

- Twice as much HSIL reported compared to SurePath.
- SurePath – similar HSIL detection reported as conventional Pap testing.
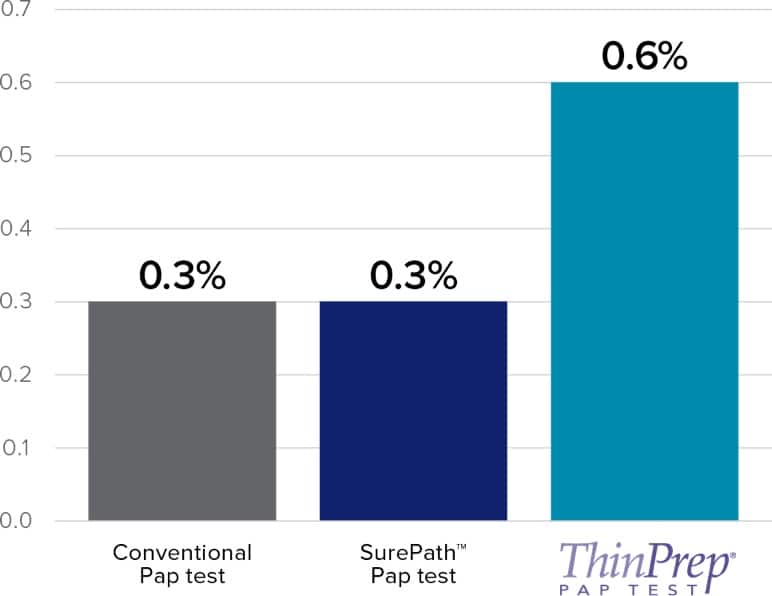
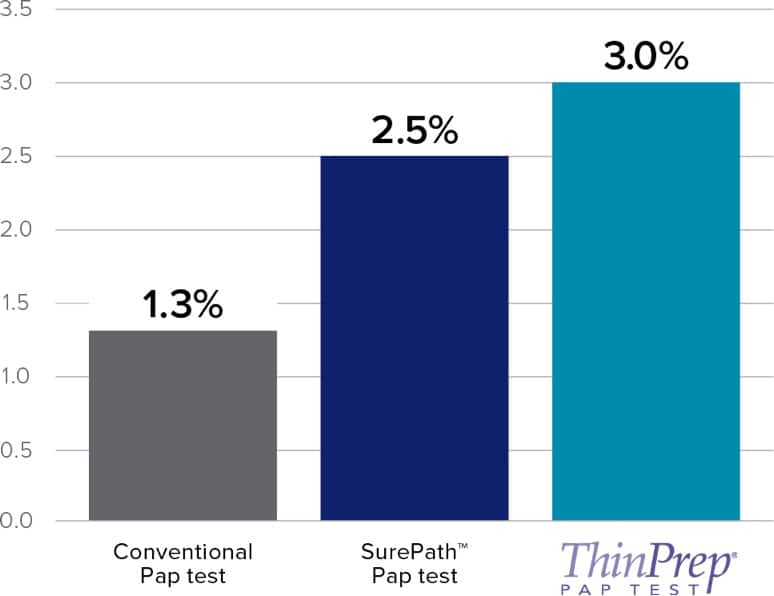
Independent Studies Show Increased LSIL and HSIL Cytology Categorization vs Manual ThinPrep Pap Test
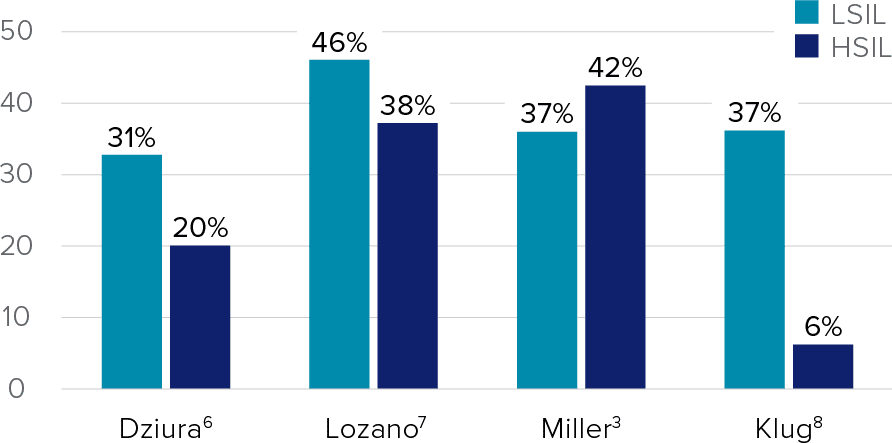
This chart is a representation of clinical data from multiple published sources. The clinical studies represented within these sources were conducted using different study designs with various assays.
Refer to the ThinPrep Imaging System Instructions for Use for results of FDA approved studies.
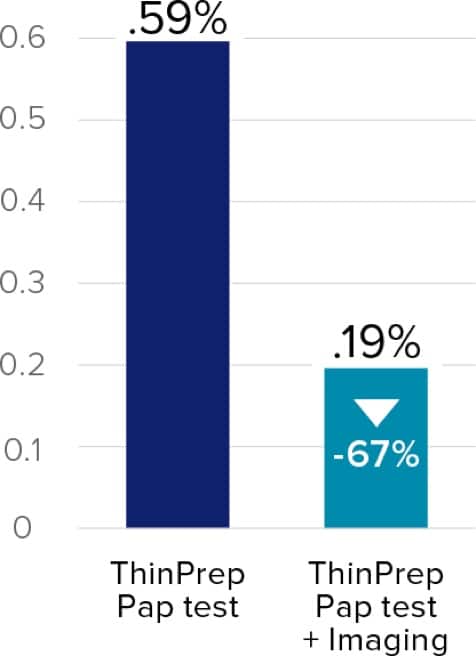
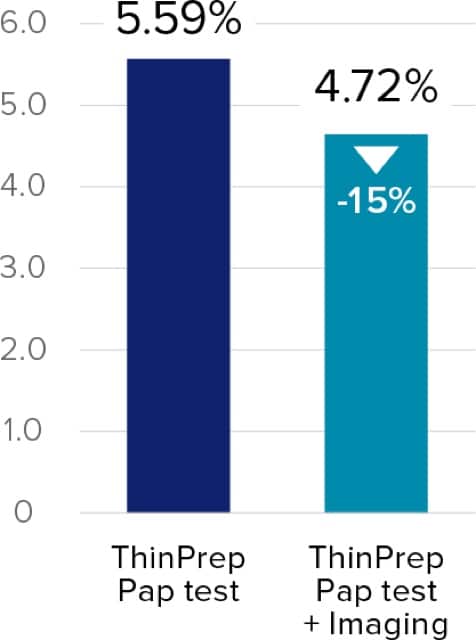
A thorough approach to cervical cancer detection
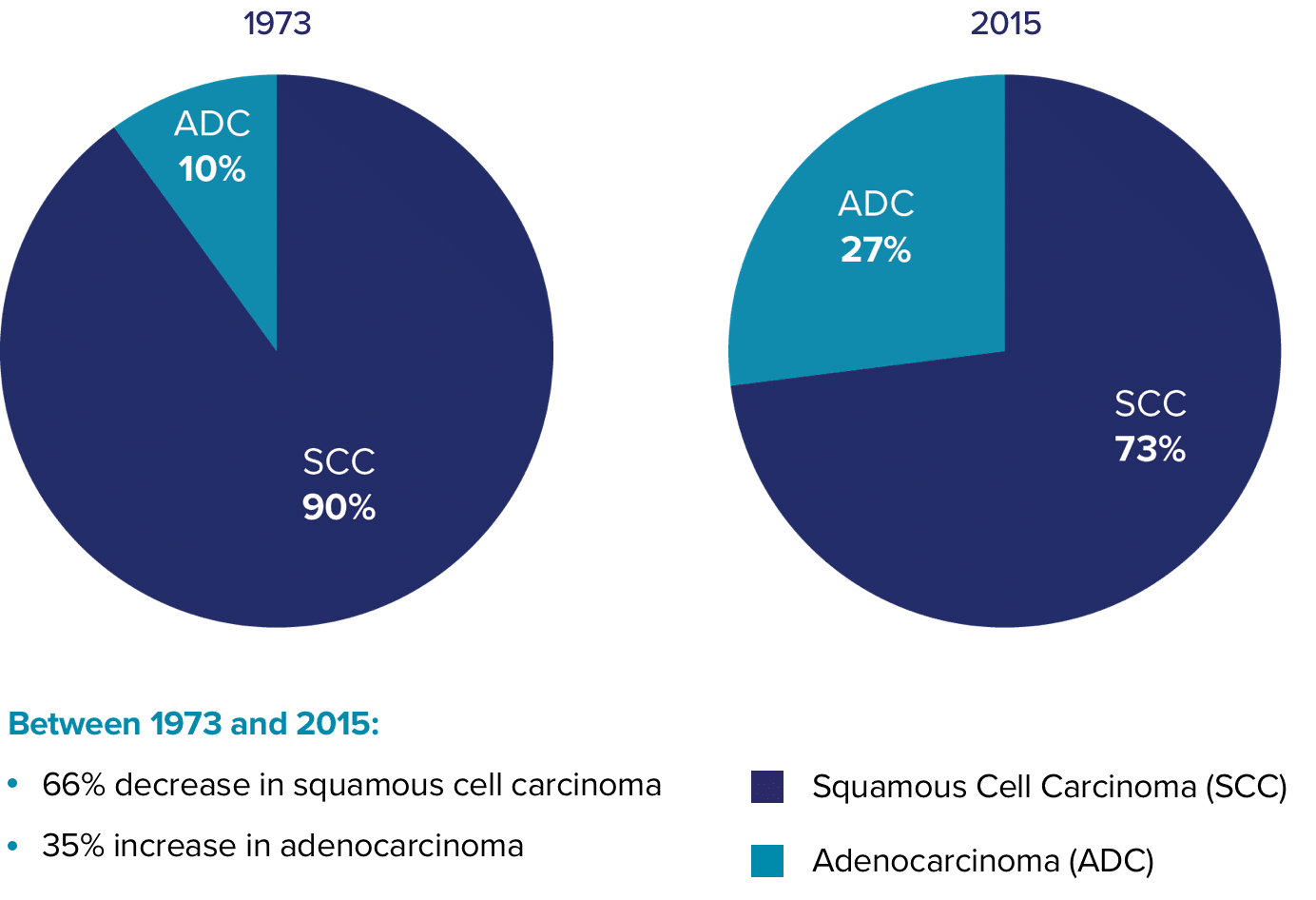
Addressing a dangerous threat
The ThinPrep® Pap test is the only Pap test with FDA-approved labeling citing multiple peer-reviewed publications supporting increased glandular disease detection compared to the conventional pap. 11-16