Aptima® Trichomonas Vaginalis Test (ATV)
The most common curable STI
Despite being the most common curable STI, Trichomonas vaginalis (TV) is as prevalent as Chlamydia (CT) and Gonorrhea (NG) combined.1 The latest CDC estimates reported more than 26 million new sexually transmitted infections in 2018, which includes 6.9 million incidences from trichomoniasis.1 Many different conditions may cause symptoms similar to TV, and co-infections can be common, making accurate diagnosis imperative for effective TV treatment.2-9
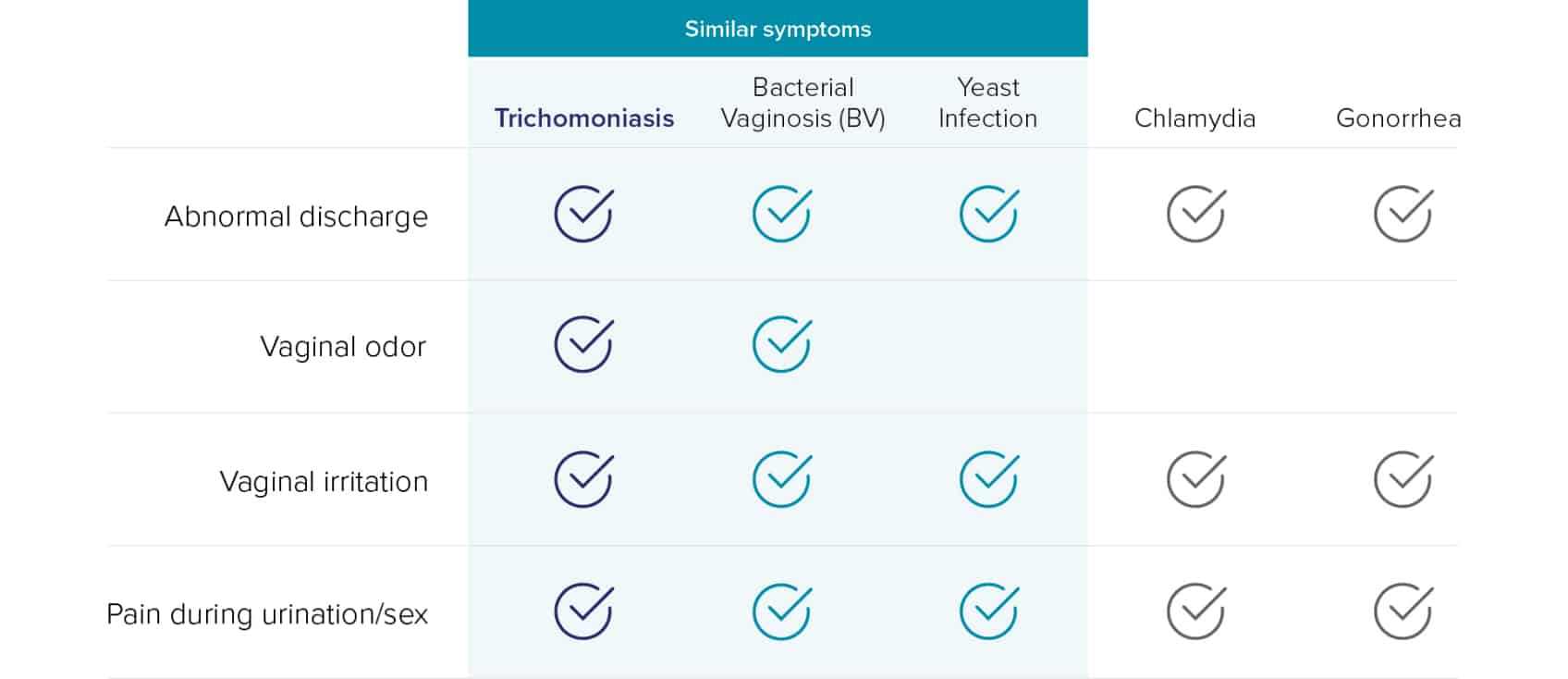
Up to 45% of trichomoniasis infections are missed by wet mount10
Wet mount testing results can be unreliable due to the following reasons11,12 :
1. Sensitivity and Timing
- Relies on detecting motile forms, leading to potential false negatives.
- Delayed testing reduces accuracy as trichomonas motility decreases over time.
2. Interpretation Challenges
- Variability in clinician skills affects accuracy.
- Non-motile forms may be confused with vaginal cells.
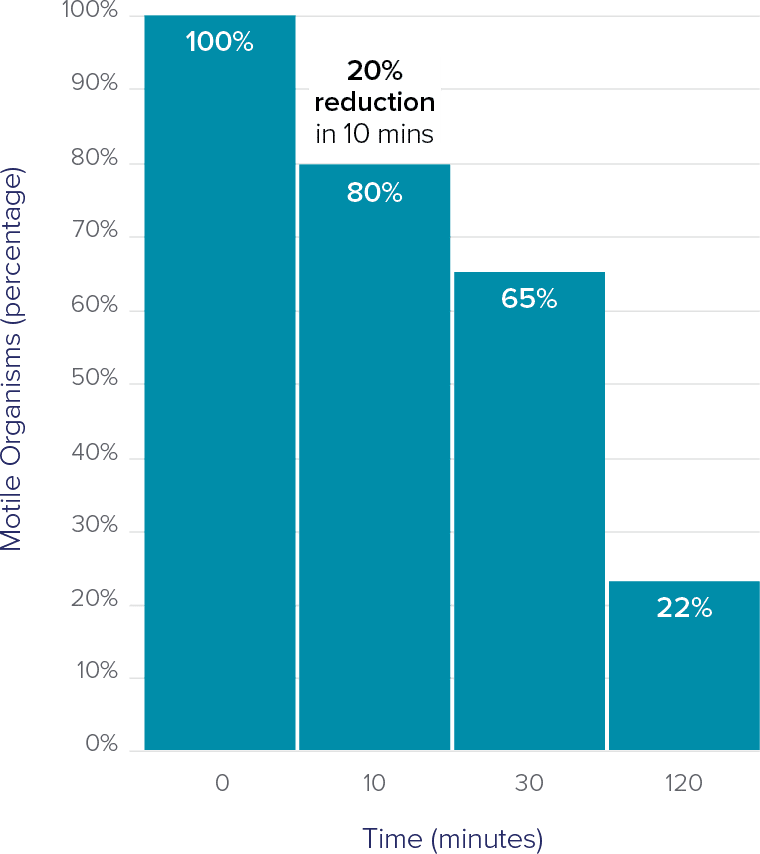
Motile organisms decrease rapidly13
Nucleic acid amplification testing (NAAT) is recommended for the diagnosis of trichomoniasis.13
ACOG
NAATs testing are highly sensitive, detecting more T. vaginalis infections than wet-month microscopy.4
CDC
Current ACOG and CDC Guidelines for TV4,13
- Nucleic acid amplification testing (NAAT) is recommended for the diagnosis of TV.
- Patients should be retested approximately 3 months after treatment because of the high rates of infection recurrence.
- For persistent infections in women, resistance testing should be considered.
- Current partners should be referred for presumptive treatment to avoid reinfection.
Additional CDC Guidance4
- All women seeking care for vaginal discharge or who report other symptoms should be tested for TV.
- All women with HIV infection are recommended to be tested.
- Women receiving care in high-prevalence settings (e.g., STD clinics and correctional facilities) should consider screening.
- Asymptomatic persons at increased risk for infections should consider screening.
- Women with TV should be tested for other STIs, including HIV, syphilis, chlamydia and gonorrhea.
Accurate TV detection, with or without symptoms
Performance data by sample type15
| Specimen Type† | Sensitivity | Specificity |
|---|---|---|
| Aptima® Multitest Swab (Clinician Collected Vaginal Sample) | 100.0% | 98.2% |
| Aptima® Multitest Swab (Patient Collected Vaginal Sample) | 98.8% | 99.4% |
| Aptima® Unisex Swab(Endocervical Sample) | 100.0% | 98.1% |
| ThinPrep® Vial | 100.0% | 98.6% |
| Aptima® Urine(Female Sample) | 100.0% | 100.0% |
| Aptima® Urine(Male Sample) | 100.0% | 99.8% |